
도터, 생분해성스텐트(BRS) 임상 환자 모집 완료
생분해성스텐트(BRS) 임상 환자 128명 모집 완료.
고위험 동맥경화반의 분자적 특성을 밝히는 융합 영상기기 상용화로
110억원 규모 시리즈B 투자 유치 성공.
A pioneer in cardiovascular intervention for 25 years, Medinol raises the bar once again with the introduction of the EluNIR elastomeric Drug-Eluting Stent — a category-of-one innovation purposefully designed to meet the unique demands of the the ever-changing vascular environment.
Dotter has launched this innovative device in the Korean market by acquiring the rights to sell the product from Medinol.


생분해성스텐트(BRS) 임상 환자 128명 모집 완료.
고위험 동맥경화반의 분자적 특성을 밝히는 융합 영상기기 상용화로
110억원 규모 시리즈B 투자 유치 성공.

Benetis Lifetime Model B Receives MFDS Approval for Precise Lesion Observation
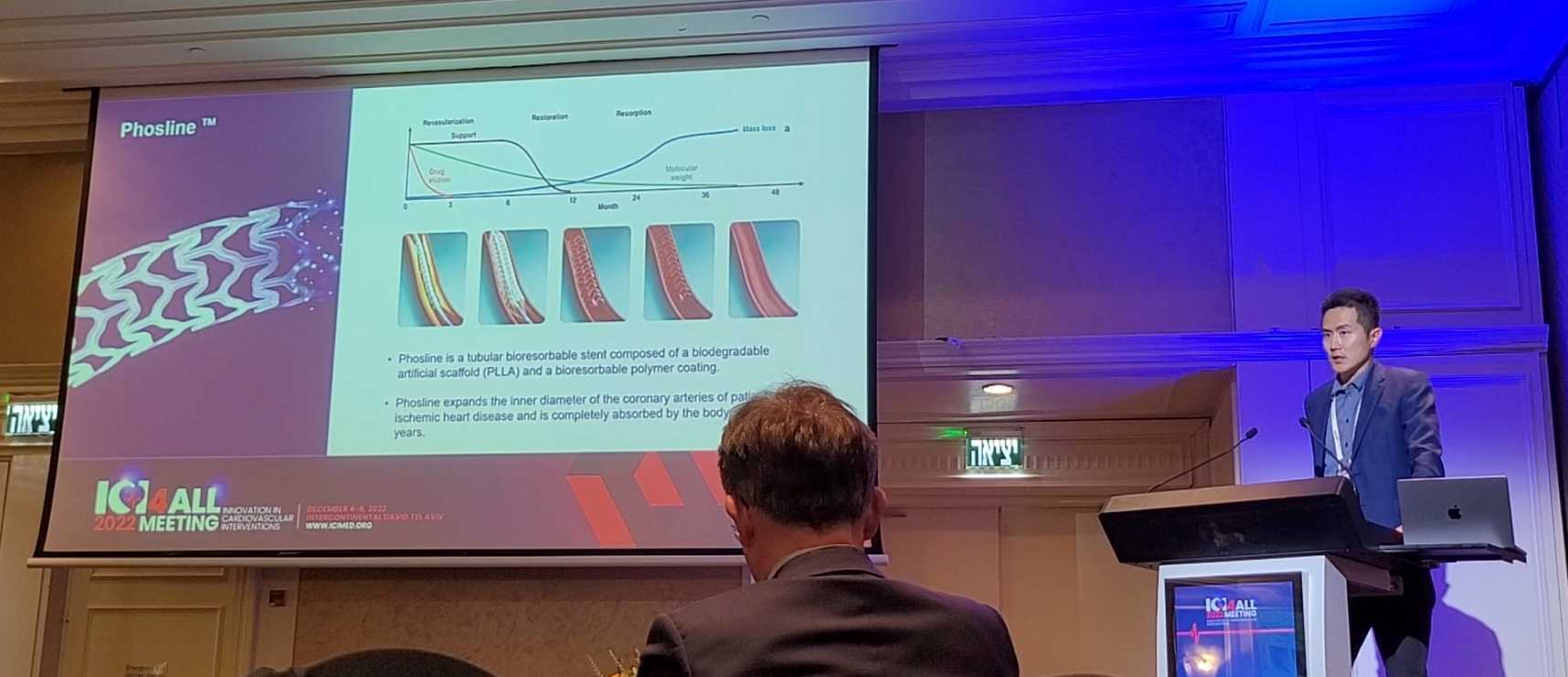
At the ICI held in Israel, Hyung-il Kim, CEO of Dotter Inc. gave a lecture on the biodegradable stent BRS

Dotter Inc. acquired the exclusive domestic rights to EluNIR™ through a recent contract with Israeli company Medinol.
EluNIR™ is scheduled to be marketed in Korea early next year through Dotter.
심장 급사의 원인이 되는 심혈관 협착증의 예방 및 치료를 위해 스텐트(Stent)로 혈관을 넓히는 시술은 이미 일반화되었다. 하지만 기존의 금속 재질 스텐트가 체내에 장기간 자리하면서 발생할 수 있는 부작용도 지적되고 있다. 도터는 이러한 우려를 해소할 수 있는 생분해성 스텐트(Bioresorbable Stent, BRS) 솔루션을 선보였다.

[Ulsan-Newsis] Reporter Hyun-cheol Cho = Ulsan Hospital announced on January 27 that it had performed the first coronary intervention in the country to insert a more improved absorbable stent for a 50-year-old male angina patient at the Cardiocerebrovascular Center on January 20.

The Korea Health Industry Development Institute (President Deok-cheol Kwon), along with the domestic venture investment platform KDB NextRound, held the ‘2019 5th K-BIC STAR DAY’ (hereafter K-BIC Star Day) held at the IR Center of the KDB in Yeouido on the 1st. It was announced on the 2nd that it was successfully held.
info@dotter.com
Inquiries about products or suggestions for partnerships
are always welcome
phone +82.32.258.5448
Fax +82.32.258.5449