Get In touch
info@dotter.com
Inquiries about products or suggestions for partnerships
are always welcome
phone +82.32.258.5448
Fax +82.32.258.5449
BRS™ is an innovative next-generation stent. Because of our proprietary V-shaped stent design, it can perform a high radial force with thin struts. Preclinical studies have shown that BRS™ holds the largest room for blood flow after treatment, in contrast to competitors’ fully bioresorbable stents.

Our new 3rd generation BRS™ is a temporary coronary scaffold comprising a fully bioresorbable scaffold (BRS) and a coated drug to block cell proliferation.BRS™ improves the coronary luminal diameter in patients with ischemic heart disease and is completely resorbed by the body in approximately 3 years.
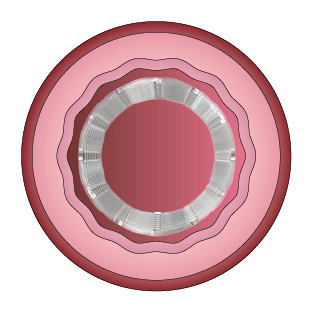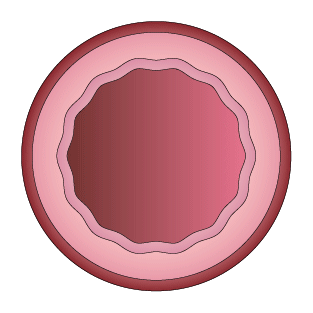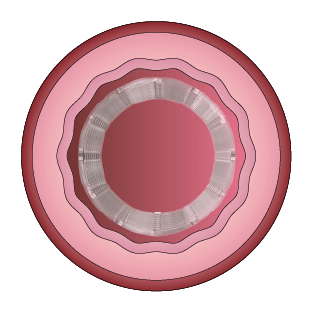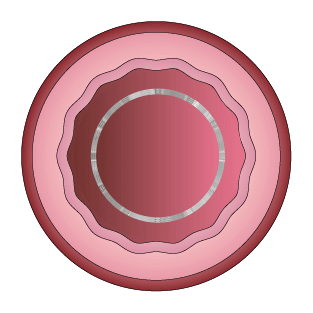
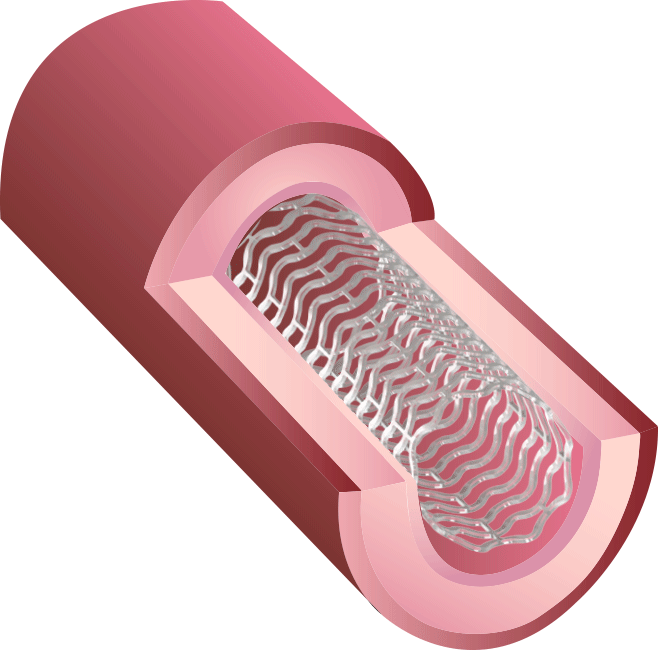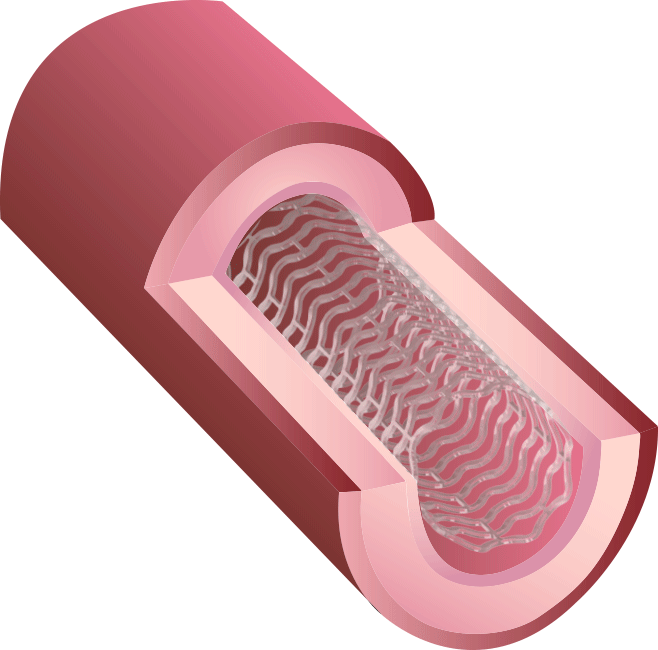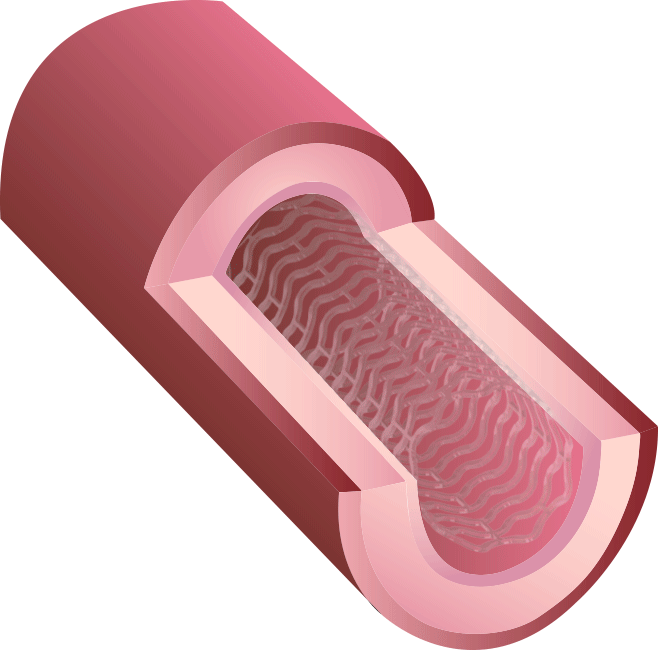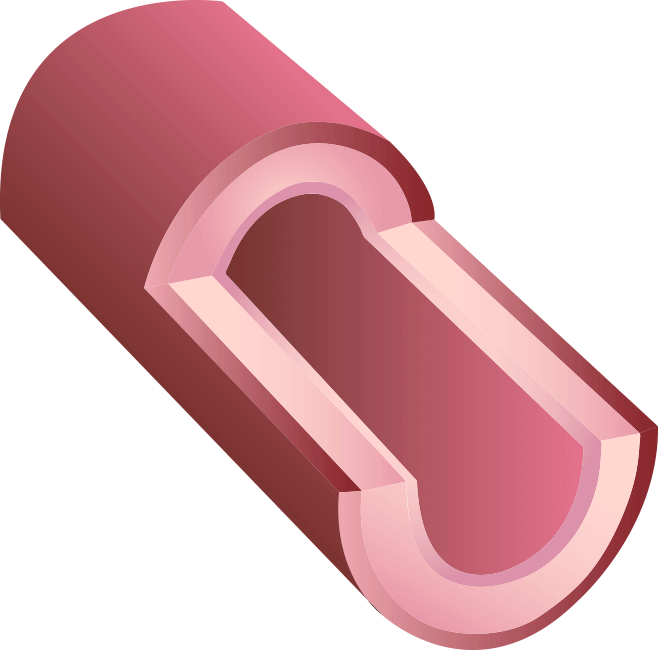
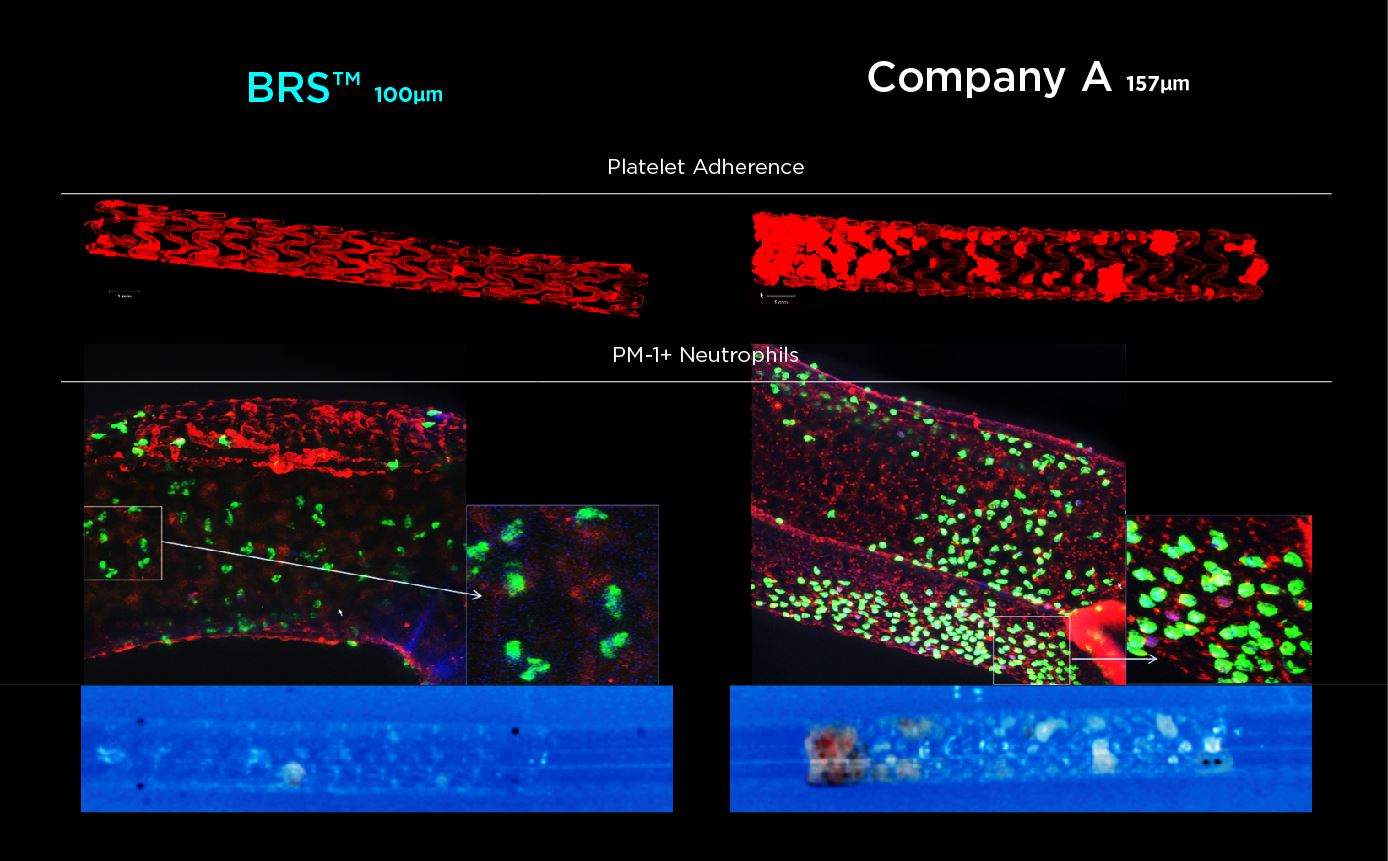
This study was designed to compare the tendency of BRS and Company A to cause platelet adhesion, thereby resulting in blood clot formation (thrombosis). BRS and Company A were deployed in custom-fabricated Sylgard tubing connected to an acute extracorporeal arteriovenous (AV) carotid shunt model.
In patients with ischemic heart disease, BRS ™ is completely absorbed by the body approximately 3 years after dilating the inner diameter of the coronary artery.
The next-generation model stent is based on patented technology that applies a PC coating to the surface, reducing the rejection of the stent and stable release of the drug.
Because the strut thickness of the stent is as thin as 100 μm, it does not interfere with blood flow; therefore, it is safe for patients and shows good treatment progress.
Dotter’s patented laser processing technology creates a clean cut surface without weakening the bioresorbable polymer, resulting in greater radial force.
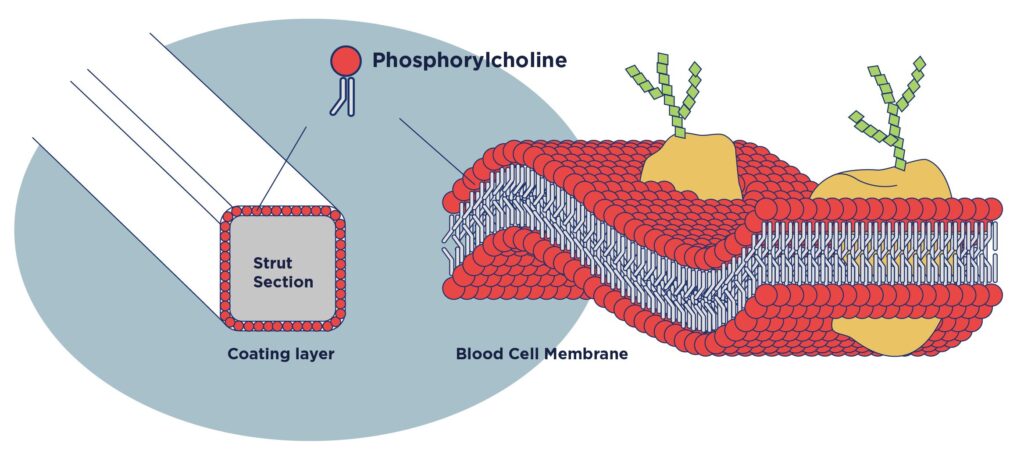
The bioresorbable polymer contains a PC group, which accounts for 99% or more of the cell membranes outside red blood cells. Unlike other hydrophilic polymers, the PC group can eliminate interactions with water.
With Dotter’s proprietary technology, the PC group can be distributed evenly over scaffold surfaces to ensure ideal and effective drug release.
The surface of the stent strut was coated with MPC polymer to be more compatible with the blood vessel environment.
info@dotter.com
Inquiries about products or suggestions for partnerships
are always welcome
phone +82.32.258.5448
Fax +82.32.258.5449